Hauptinhalt
Topinformationen
Functional Nanoporous Membranes
Functional membranes containing well defined transport-units like carrier molecules, ion pores or channels, are able to selectively recognise and transport distinct ions or molecules. Prototypes can presumably be found in biological systems. While nature applies nearly perfect systems, virtually all industrially applied porous membranes have two things in common: the pores are spatially disordered and the pore-geometry’s are characterised by broad size distributions. Even in fundamental research on catalysis, molecular engineering, physical chemistry or advanced membrane applications there is a constantly growing demand in structurally well defined mesoporous systems. The excellently developed - and intensively reviewed - “classical“ preparation methods for mesoporous systems like powder sintering, thermal induced phase separations (TIPS), phase inversing solution casting, void formation, stretching or non templated sol-gel processes presently seem unable to meet these challenges. Track etching creates parallel and uniformly pores, but principal reasons limit the volume porosity to 15 - 17 %. An important approach toward the synthesis of well defined mesoporous materials is based on the employment of supramolecular aggregates.
We exploit the ability of small molecules to self-assemble into cylinderical superstructures with well defined diameters, but almost “infinite” lenght (cf. Supramolecular Organogels ). The self-assembled fibres can either be used as templates to create pores of uniform shape and sizes (”Gel Template Leaching ” approach) or the fibres are designed to contain a transport active channel in their interior (” Matrix Fixed Transport Channels”), cf. Figure 1.
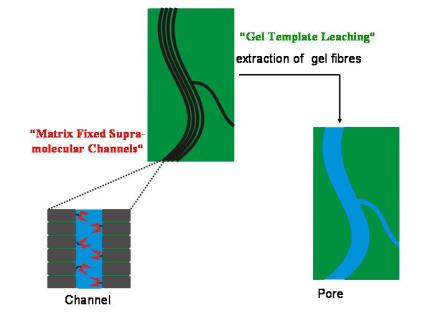
Figure 1: Schematic depiction of the two complementary strategies to generate functional porous membranes by means of self assembled fibres
1. Gel-Template Leaching as a tool to generate functional mesoporous films and membranes
In cooperation with Prof. M. Möller DWI at the RWTH-Aachen, Pauwelsstr. 7, D-52056 Aachen, Germany
Our synthetic approach to design new types of functional membranes makes use of the self-assembly of supramolecular aggregates. Low molecular type porogenes are developed, gelating organic solvents by the self-organized assembly into growth-limited cylindrical aggregates, yielding “supramolecular organogels”. After curing of the liquid matrix the low-molecular weight cylinder-network can easily be extracted, resulting in a nanoporous material. Pores with well defined diameters between 5 and 200 nm and narrow pore-size distributions can be obtained (Gel Template Leaching). Subsequently the pores inner walls can chemically be modified to yield functional, chemically selective membranes (cf. Figure 1.1).
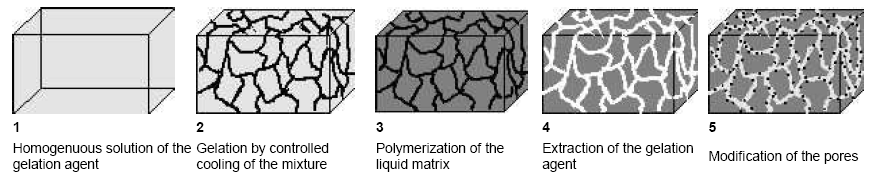
Figure 1.1: Schematic drawing of the "Gel-Template-Leaching" process
2. Crownether - functionalised Matrix - Fixed Supramolecular Transport Channels
Ion selective membranes are of considerable interest in the field of medical analytics, dialysis, construction of chemical sensors and cleaning of waste water solutions. Ion selectivity can be attained by using functional membranes, either containing „carrier” molecules or by leading the permeat through functional channels. In our approach to construct alkali metal selective membranes we want to combine both concepts: The membranes should contain functional channels, which are builded up from self organized ion selective carrier molecules. It is known that crown-ether compounds like 1,4,7,10,13-pentaoxacyclopentadecane (”15-c-5″) selectivly form complexes with alkali metal cations, the stability of the complex beeing ruled by the radius of the cation [2]. In an earlier report it was shown, that crown ether containing compounds like 2-hydroxymethyl-[1,4,7,10,13-pentaoxabenzocyclopentadecane]-3,4,5-tris[4-(n-dodecyl-1-oxy)benzyloxy]- benzoate (”DOBOB-CE”, Fig. 2.1) are able to form a hexagonal columnar mesophase. In this mesophase the DOBOB-CE molecules are stacked to columns in which the crown ether moieties form potential ion channels parallel to the column axis (cf. Fig.2.2). In fact it was shown, that DOBOB-CE exhibits ionic conductivity in the mesophase.
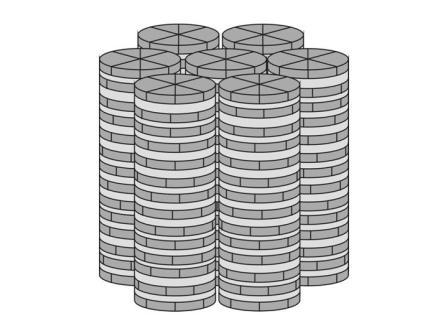
Figure 2.2: Schematical drawing of the molecular arrangements of DOBOB-CE-molecules in the hexagonal disordered columnar mesophase.
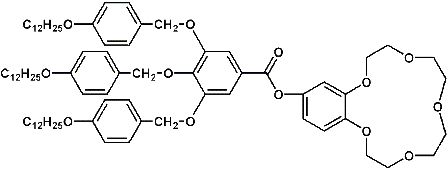
Figure 2.1: Structure Formula of DOBOB-CE
Supramolecular Transport-Channels via self-organization of small receptor molecules:
We succeeded to incorporate self-assembled networks of cylinders of DOBOB-CE into an apolar methacrylate resin. Morphological studies on gels formed by fast cooling of DOBOB-CE solutions in monomeric methacrylate mixtures, demonstrated the formation of long interdigitated cylindrical columns of DOBOB-CE with diameters of 5 - 10 nm. The structure could be fixed by UV-curing of the methacrylate resin at low temperature. Since the DOBOB-CE cylinders however were not linked chemically to the polymer matrix, they were easily leached out of the membrane by solvents and the existance of ion channels could not be demonstrated by ion selectivity measurements.
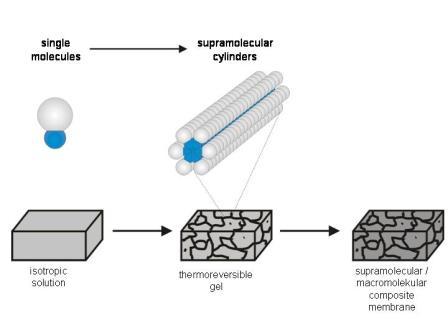
Scheme 2.1: Schematic sketch of the self-assembly of a low molecular weight amphiphilic crown-ether compound into supramolecular transport channels and a TEM picture of matrix-fixed supramolecular transport-channels
For these reasons DOBOB-CE analogues, bearing polymerisable methacrylate groups and allowing the covalent fixation of the molecules to the polymeric matrix are synthesized. Investigations concern the characterisation of the self-organization abilities of the compounds and their application for the preparation of selective membranes containing supramolecular transport channels.
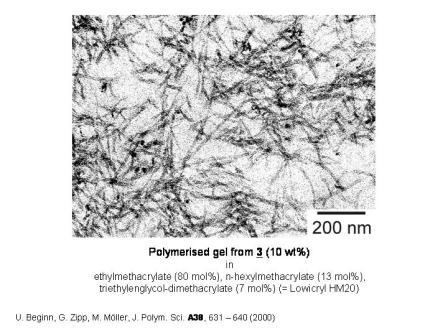
Figure 2.3: TEM micrographs of polymerised supramolecular fibres of methacrylate functionalised DOBOB-CE in a crosslinked methacrylate matrix.
Composite films, containing such self-organized supramolecular cylindrical strings will act as ion-selective membrane, since only such ions can permeate, which fit into the inner moieties of the supramolecular strings.
3. Sulfonate - functionalised Matrix - Fixed Supramolecular Transport Channels
The aim of this project is to construct membranes that contain self-organised, supramolecular transport channels with densely packed sulfonate groups along the transport path. Such well defined model systems are intended for comparative studies to reveal the effect of the molecular order within a transport path on the transport properties. Furthermore, investigations on well defined sulfonate channels may help to improve our understanding of Nafion® - ionomer membranes that are important examples of industrially applied synthetic ion-selective membranes.
In addition to their good mechanical properties and superior chemical inertness, Nafion ionomers provide an unusual degree of permeability selectivity in favour of cations over anions. This selectivity exceeds the well understood Donnan permselectivity, based on differences in the equilibrium sorption of counterions and co-ions in an ion exchanging medium. The persistence of permselectivity beyond the Donnan limit has been referred to as “superselectivity”. A “cluster-network” model (Figure 3.1) was proposed to describe the structure of these ionomers.
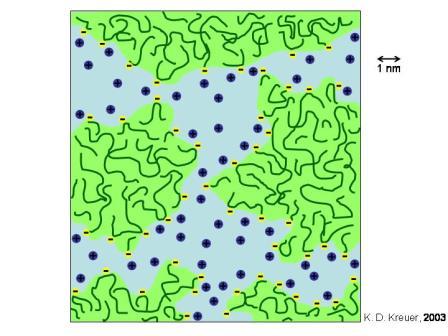
Figure 3.1: Schematic sketch of the proposed microstructure of a Nafion membrane
The polymer fixed ions and the absorbed water molecules segregate from the fluorocarbon matrix into domains, approximately 4 - 5 nm in diameter, interconnected by short narrow channels (diameter ca. 1 nm, Fig. 3.1). The fixed charges are imbedded in the water phase, close to the aqueous / fluorocarbon interface. Although the overall charge density of the membrane is comparable to other ion exchange membranes (1 - 10 meq/cm3) the local concentration of the fixed charges in the ion clusters exceed this value by far. Hence, co-ions become strongly rejected even at elevated source phase concentrations. Since the diameters of the inter-cluster channels are below the Debye-length of the permeating ions, Nafion membranes also exhibit high cation - cation selectivity, i.e. larger, more hydrophobic ions exhibit significant smaller transport rates than small hydrophilic ions. It is, however, a severe disadvantage of perfluorinated ionomer systems that the content of the cluster phase cannot arbitrarily be increased, since the ionomers become water soluble on exceeding a certain sulfonate content.
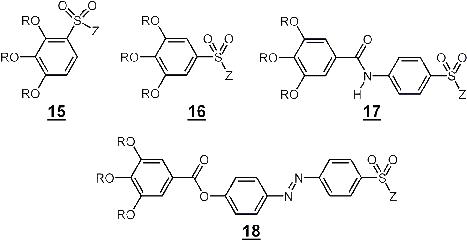
Scheme 3.1: Structure formula of self-organising sulfonate building blocks for the generation of sulfonate - supramolecular functionalised transport channels (R = H-X-, CH2=CH-X-, CH2=CH-COO-X-, X = -(CH2)n-), Z = -NH2, Alkali metal)
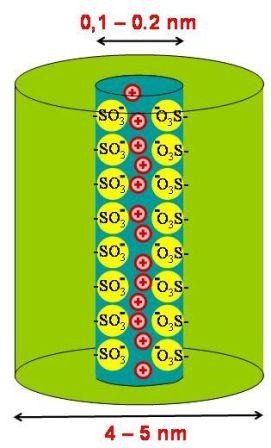
Figure 3.2: Schematic drawing of the aimed structure of a sulfonate transport channel

